*This information was originally published as part of an abstract for the ACR Convergence 2025*
Understanding Rheumatoid Arthritis and the Unmet Patient Need
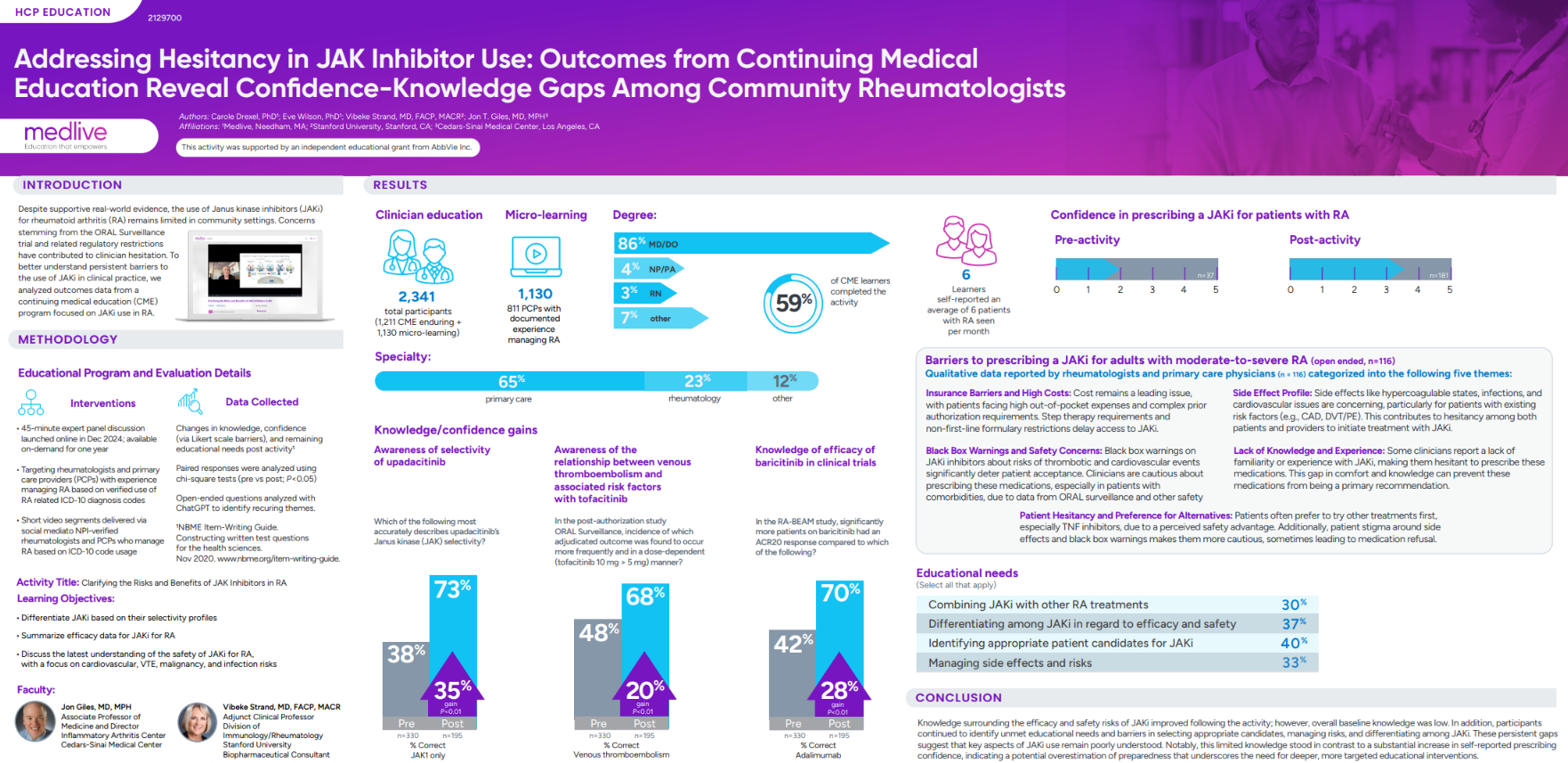
While Janus kinase inhibitors (JAKi) have demonstrated efficacy in rheumatoid arthritis (RA), real-world adoption remains limited, particularly in community settings. Persistent concerns following the ORAL Surveillance trial and resulting regulatory restrictions have contributed to clinician hesitation. These challenges highlight the need for targeted education to help rheumatologists and primary care providers accurately evaluate patient candidacy, differentiate among JAK inhibitors, and manage associated safety risks.
Understanding the balance between efficacy and risk remains a critical need. As new data clarify the safety and selectivity profiles of JAKi, clinicians require guidance to interpret emerging evidence, communicate risk–benefit considerations, and support shared decision-making with patients who may be hesitant to initiate therapy.
The Medlive Approach
To address these ongoing barriers, Medlive, in collaboration with leading rheumatology experts from Cedars-Sinai Medical Center and Stanford University, developed an accredited, outcomes-driven CME program titled Clarifying the Risks and Benefits of JAK Inhibitors in RA. This 45-minute expert panel discussion was designed to improve clinical decision-making and reduce uncertainty surrounding JAKi use.
The program engaged rheumatologists and primary care providers with verified RA experience, combining live and enduring online formats with short micro-learning videos distributed via social media to NPI-verified clinicians. The activity focused on three core learning objectives: differentiating JAKi by their selectivity profiles, summarizing efficacy data, and discussing the latest evidence on safety, including cardiovascular, thrombotic, malignancy, and infection risks.
Key Findings
- Persistent barriers and needs: Clinicians cited several factors limiting the use of JAK inhibitors, including insurance and cost challenges, safety concerns related to black box warnings, and limited firsthand prescribing experience. The most frequently reported educational needs involved identifying appropriate patient candidates (40%), differentiating among JAK inhibitors (37%), and managing treatment-related side effects (33%).
- Significant knowledge gains: Although baseline understanding of JAK inhibitor efficacy and safety was low, participants demonstrated meaningful improvements following education. Correct responses increased by 35% for JAK selectivity, 20% for thromboembolism risk factors, and 28% for JAK inhibitor efficacy (p<0.01 across all measures), reflecting strong gains in clinical comprehension.
- Confidence–knowledge gap identified: Despite measurable learning gains, participants continued to report uncertainty in patient selection, risk management, and differentiation among JAK inhibitors. This contrasted with a notable rise in self-reported prescribing confidence, suggesting potential overestimation of readiness and reinforcing the need for continued, targeted education to strengthen clinical decision-making.
Conclusion – Collaborating for Improved Patient Outcomes
The outcomes of this activity reveal an important opportunity to strengthen clinician understanding of JAK inhibitor use in RA. While participants showed significant gains in knowledge and confidence, persistent gaps in clinical interpretation and patient selection underscore the need for continued, case-based education.
By addressing misconceptions and aligning clinician confidence with evidence-based competence, Medlive’s educational approach supports more informed prescribing decisions and improved patient communication around JAK inhibitor therapy.
Continued collaboration between educators, clinical experts, and industry partners remains essential to closing the confidence–knowledge gap and advancing optimal use of JAK inhibitors in the management of rheumatoid arthritis.
To learn more about partnering with Medlive to develop impactful CME programs, reach out via our Contact Us page.
This activity was supported by an independent educational grant from AbbVie Inc.




