*This information was originally published as part of an abstract for the 2025 San Antonio Breast Cancer Symposium (SABCS)*
Understanding Triple-negative Breast Cancer (TNBC) and the Unmet Patient Need
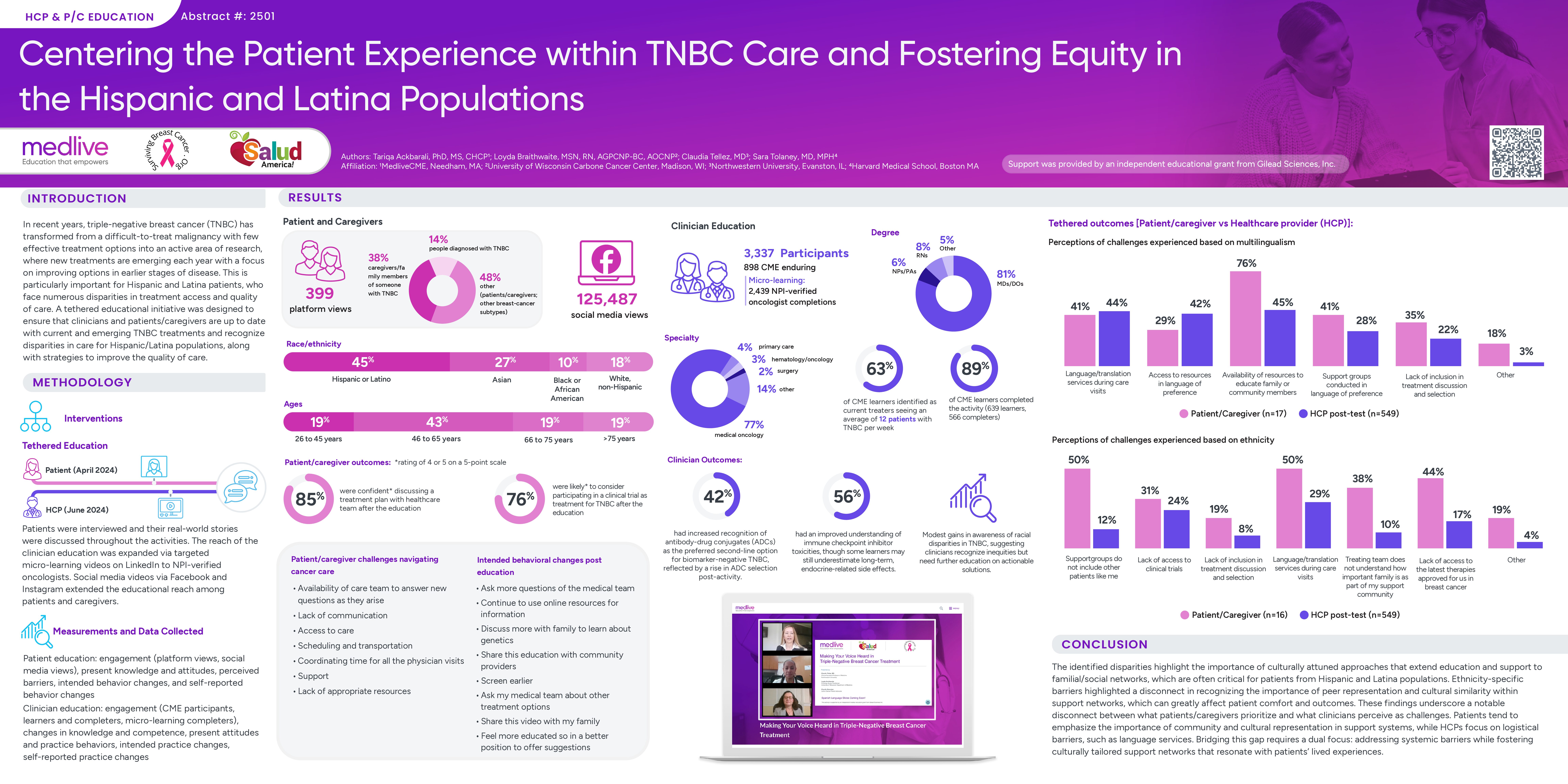
Triple-negative breast cancer (TNBC) has rapidly evolved from a malignancy with limited treatment options to an area of active therapeutic innovation, particularly for earlier stages of disease. Yet persistent inequities remain, especially for Hispanic and Latina patients, who face disproportionate barriers in diagnosis, treatment access, and supportive care.
Patients and caregivers from these communities often navigate complex health systems while also contending with cultural, linguistic, and socioeconomic challenges that influence care engagement and outcomes. As new TNBC therapies emerge, aligning clinical decision-making with culturally attuned communication, peer representation, and meaningful caregiver involvement remains essential to improving equity in care delivery.
The Medlive Approach
To address these disparities, Medlive partnered with Salud America! and Surviving Breast Cancer.org to design a tethered educational initiative that delivered aligned learning experiences for clinicians and patients/caregivers. The goal was to improve awareness of current and emerging TNBC treatments, illuminate disparities affecting Hispanic and Latina patients, and provide actionable strategies to strengthen communication and shared decision-making.
Patient education (April 2024) featured real-world stories captured through interviews, emphasizing cultural identity, community support, and the lived experience of navigating cancer care. Social media videos delivered via Facebook and Instagram further broadened engagement.
Clinician education (June 2024) included a CME activity supported by micro-learning videos delivered to NPI-verified oncologists through LinkedIn, focusing on therapeutic innovation, toxicity management, and the recognition of inequities in treatment access and quality of care.
This dual-audience design—highly coordinated across platforms—ensured that both patients/caregivers and clinicians received complementary guidance to strengthen communication, improve trust, and support equitable treatment pathways.
Key Findings
- High patient and caregiver engagement: In all, 3,337 clinicians and caregivers participated in the initiative, with 898 completing CME activities and 2,439 engaging through micro-learning, reflecting high interest and broad applicability across the TNBC care continuum.
- Increased clinical readiness: Post-education, 56% of clinicians demonstrated improved understanding of immune checkpoint inhibitor toxicities, and 42% recognized antibody-drug conjugates (ADCs) as preferred second-line options for biomarker-negative TNBC.
- Behavioral shifts: After the program, patients intended to ask more questions, discuss genetic risks with family, and explore clinical trial options. Clinicians reported increased awareness of inequities and a need for more actionable strategies to support Hispanic and Latina patients.
Conclusion: Advancing Equity Through Patient- Centered Education
Findings revealed a significant gap between what Hispanic and Latina patients value in their care experience and what clinicians perceive as barriers. Patients prioritized cultural representation, peer support, and family involvement, while clinicians emphasized structural and language-related challenges.
By grounding education in lived experiences and reinforcing it with clinical data, this initiative demonstrated that culturally attuned approaches can strengthen communication, enhance trust, and support more equitable TNBC care. Ongoing focus on community-linked navigation, peer-informed resources, and language-accessible education will be essential to addressing disparities and improving outcomes for Hispanic and Latina patients with TNBC.
To learn more about partnering with Medlive to develop impactful CME programs, reach out via our Contact Us page.
This initiative was supported by an independent educational grant from Gilead Sciences, Inc.